221783
Copper(II) chloride dihydrate
reagent grade
Synonym(s):
Cupric chloride dihydrate
Select a Size
About This Item
grade
reagent grade
Quality Level
vapor density
>1 (vs air)
form
crystals
concentration
35.0-38.0% Cu (EDTA titration)
pH
3-3.8
mp
100 °C (dec.) (lit.)
SMILES string
Cl[Cu]Cl.[H]O[H].[H]O[H]
InChI
1S/2ClH.Cu.2H2O/h2*1H;;2*1H2/q;;+2;;/p-2
InChI key
MPTQRFCYZCXJFQ-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- As a recoverable promoter for the conversion of oximes to carbonyl compounds under mild reaction conditions.
- To catalyze Biginelli condensation of aldehyde, β-ketoester and urea or thiourea to form dihydropyrimidinones under solvent-free and microwave irradiations.
- Along with stannous chloride for the allylation of aldehydes.
signalword
Danger
Hazard Classifications
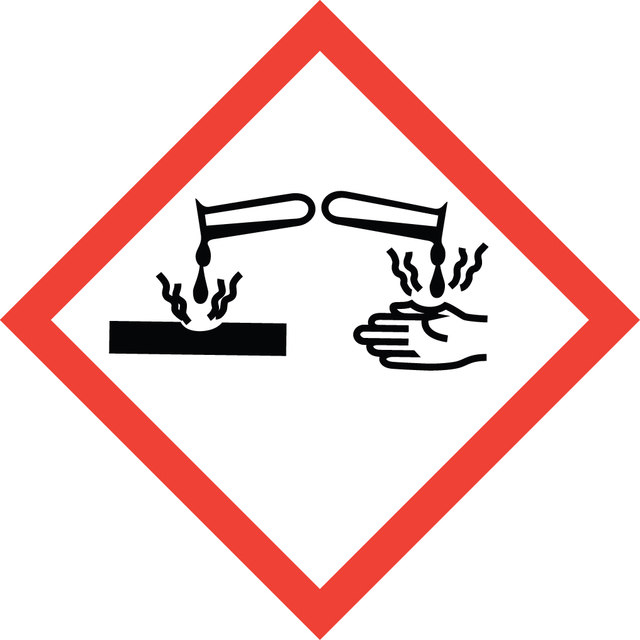
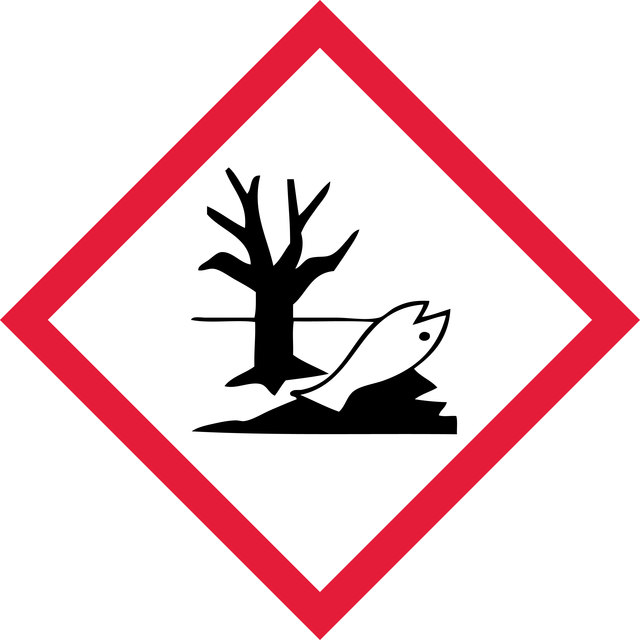
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service