679011
2-(Diphenylphosphino)terephthalic acid 1-methyl 4-pentafluorophenyl diester
97%
Synonym(s):
1-Methyl-4-(pentafluorophenyl)-2-(diphenylphosphino)-1,4-benzenedicarboxylate
Sign Into View Organizational & Contract Pricing
Select a Size
About This Item
Empirical Formula (Hill Notation):
C27H16F5O4P
CAS Number:
Molecular Weight:
530.38
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Quality Level
assay
97%
form
solid
reaction suitability
reaction type: click chemistry
mp
109-111 °C
storage temp.
−20°C
SMILES string
COC(=O)c1ccc(cc1P(c2ccccc2)c3ccccc3)C(=O)Oc4c(F)c(F)c(F)c(F)c4F
InChI
1S/C27H16F5O4P/c1-35-27(34)18-13-12-15(26(33)36-25-23(31)21(29)20(28)22(30)24(25)32)14-19(18)37(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-14H,1H3
InChI key
OURNVXDJALDDIG-UHFFFAOYSA-N
Related Categories
Application
- Staudinger ligation reagent for the conjugation of amine and azide containing compounds or biomolecules.
- The amine functionalized molecule first reacts with the phosphine through the activated pentafluorophenyl ester.The azide-molecule is then reacted with the newly labeled phosphine to form the iminophosphorane, and the aza-ylide is subsequently captured by the methyl ester to yield the covalent conjugated product.
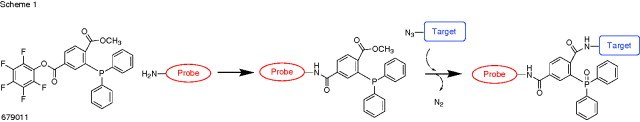
The Staudinger Ligation: A High-Yield, Chemoselective, and Mild Synthetic Method
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Saxon et al.
Science (New York, N.Y.), 287(5460), 2007-2010 (2000-03-17)
Selective chemical reactions enacted within a cellular environment can be powerful tools for elucidating biological processes or engineering novel interactions. A chemical transformation that permits the selective formation of covalent adducts among richly functionalized biopolymers within a cellular context is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service