209139
Silver nitrate
ACS reagent, ≥99.0%
동의어(들):
Nitric acid silver(I) salt
로그인조직 및 계약 가격 보기
크기 선택
제품정보 (DICE 배송 시 비용 별도)
Linear Formula:
AgNO3
CAS 번호:
Molecular Weight:
169.87
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.21
Grade:
ACS reagent
Grade
ACS reagent
Quality Level
vapor density
5.8 (vs air)
분석
≥99.0%
양식
solid
반응 적합성
reagent type: catalyst
core: silver
용액 투명도
passes test
불순물
Free acid, passes test
≤0.01% not HCl pptd.
mp
212 °C (dec.) (lit.)
음이온 미량물
chloride (Cl-): ≤5 ppm
sulfate (SO42-): ≤0.002%
양이온 미량물
Cu: ≤2 ppm
Fe: ≤2 ppm
Pb: ≤0.001%
SMILES string
[O-][N+]([O-])=O.[Ag+]
InChI
1S/Ag.NO3/c;2-1(3)4/q+1;-1
InChI key
SQGYOTSLMSWVJD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Silver nitrate, also known as Nitric acid silver(I) salt, is an odorless, colorless, or white versatile inorganic compound widely used as an oxidant and cauterizing agent due to its sclerosing, dehydrating, and escharotic properties. It is commonly used as a catalyst in the hydrolytic oxidation of organosilanes, leading to the production of high hydrogen yield and organosilanols.
애플리케이션
Silver nitrate can be used as a catalyst:
It can be used to prepare some supported acid catalysts for various organic transformations. For example:
AgNO3 is also used as a precursor salt to synthesize silver nanoparticles.
- For the Friedel-Crafts acylation of benzene derivatives to synthesize corresponding ketones.
- In the hydrolytic oxidation of organosilanes to produce hydrogen.
It can be used to prepare some supported acid catalysts for various organic transformations. For example:
- AgNO3–SiO2 based catalyst is used to dearomatize spirocyclization.
- Polyaniline-AgNO3-PTSA (para-toluene sulfonic acid) catalyst is used to synthesize anti-β-amino ketones via the three-component Mannich reaction of aromatic aldehydes, aromatic amines, and cyclohexanone.
AgNO3 is also used as a precursor salt to synthesize silver nanoparticles.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
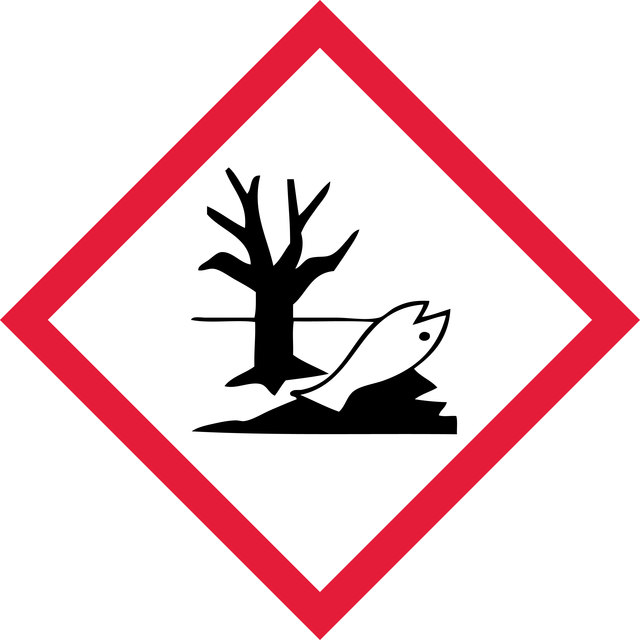
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Ox. Sol. 2 - Repr. 1B - Skin Corr. 1A
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Catalytic hydrogen evolution from hydrolytic oxidation of organosilanes with silver nitrate catalyst
Teo AKL and Fan WY
Royal Society of Chemistry Advances, 4(71), 37645-37648 (2014)
Convenient Method for the Friedel-Crafts Acylation of Benzene Derivatives Using Silver Nitrate as Catalyst
Rai KML, et al.
Synthetic Communications, 41(7), 953-955 (2011)
Hongyan Yang et al.
Antibiotics (Basel, Switzerland), 9(11) (2020-11-01)
Antimicrobial resistance is currently an important concern, but there are few data on the co-presence of metal and antibiotic resistance in potentially pathogenic Escherichia coli entering the food chain from pork, which may threaten human health. We have examined the
Donna Dang et al.
Molecular carcinogenesis, 56(11), 2474-2485 (2017-06-16)
Calcification of the breast is often an outward manifestation of underlying molecular changes that drive carcinogenesis. Up to 50% of all non-palpable breast tumors and 90% of ductal carcinoma in situ present with radiographically dense mineralization in mammographic scans. However
D D Songstad et al.
Plant cell reports, 7(4), 262-265 (1988-06-01)
The effect of the ethylene antagonists norbornadiene and silver nitrate and the ethylene precursor l-aminocyclopropane-l-carboxylic acid (ACC) on Zea mays plant regeneration was studied. A 12-fold increase in plant regeneration, as measured by number of plants obtained per gram fresh
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.