Retention Behavior of Mono- and Disaccharides on a Supel™ Carbon LC Column
Andras Z. Komaromy1, Edward S.X. Moh1, Nicolle H. Packer1, Morten Thaysen-Andersen1
1School of Natural Science, Macquarie University, Sydney, Australia
Abstract
While the use of porous graphitised carbon (PGC) liquid chromatography (LC) columns for the separation of common N- and O-linked glycans ranging from 4-15 saccharide residues has been successful in many respects, it remains challenging to profile shorter glycans (mono- and disaccharides) in the same chromatographic run due to their relatively poor retention characteristics on conventional PGC-LC columns. In this work, we investigated the retention behaviour of two hexose (Hex) monosaccharide isomers i.e. fructose (Fru) and glucose (Glc) as well as three hexose disaccharide (Hex2) isomers i.e. maltose (Mal), lactose (Lac) and sucrose (Suc) on a Supel™ Carbon LC column using a high-resolution Q-ToF mass spectrometer as the detector. We demonstrate that the PGC column was able to retain the non-reduced and reduced forms of all compounds on a typical glycomics gradient using conventional solvents. Chromatographic analyses of non-reduced and reduced Fru, Glc, Mal, Lac and Suc showed reproducible retention patterns over triplicate chromatographic runs of all samples demonstrating robustness of the PGC column for the retention of various mono- and disaccharides.
Notably, non-reduced Glc exhibited a stronger retention potential on the Supel™ Carbon LC column than non-reduced Fru shedding light on the role of the aldo and keto position of the double bonded oxygen. Interestingly, reduced Fru eluted later than non-reduced Fru while reduced and non-reduced Glc displayed the opposite behavior.
Reduced Mal and Lac eluted nearly one minute earlier than their corresponding non-reduced disaccharides demonstrating that these species can readily be separated using the Supel™ Carbon LC column. In contrast, only relatively weak separation was achieved for the reduced and non-reduced forms of Mal and Lac under the tested conditions. The analysis of their mixtures are key experiments required to confirm this preliminary conclusion.
In summary, our findings show a potential of the Supel™ Carbon LC column for reproducible mono- and disaccharide analysis, which previously has been considered challenging using LC-MS/MS.
Introduction
Fru is a ketohexose with a double bonded oxygen atom on the second carbon atom in the linear form of the molecule (Figure 1). The monosaccharide is mostly found in plants where it is often bound to Glc forming the disaccharide sucrose.1, 2 Although Fru is not a glycan-forming monosaccharide, it is a structural isomer of Glc and can be converted to a sugar alcohol by reductive beta-elimination. Therefore, comparing its retention behavior to Glc is justified as it might shed light on the role of the spatial location of the double bonded oxygen in the non-reduced form and the -OH group in the reduced form in the interaction with the PGC column.
Mal, Lac and Suc are the three common dietary Hex disaccharides (Hex2) having the same chemical formula and all containing a Glc constituent. Mal and Lac are reducing sugars because they can present a free aldehyde group while Suc is a non-reducing disaccharide (Figure 1). Mal is composed of two D-glucoses linked by an α→14 glycosidic bond. The disaccharide usually arises from hydrolysis of starch and serves as one of the main sources of Glc.3 Lac is formed by D-galactose (Gal) and D-Glc bound via β→14 glycosidic bond and it is one of the main constituents of human and animal milk. Lac has two isomeric forms, α- and β-Lac, which differ in their steric configuration of the hydroxyl group of the C-1 moiety of Glc.4 Suc plays a unique role in the human diet. This disaccharide contains D-Fru and α-D-Glc subunits linked through a glycosidic bond between C1 of α-Glc to C4 of β-Fru.2
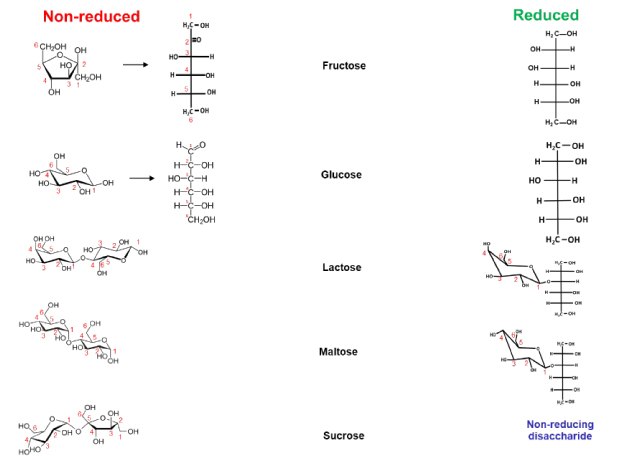
Figure 1.Overview of two Hex monosaccharide isomers (Fru, Glc) and three Hex2 disaccharide isomers (Lac, Mal, Suc) investigated in this study. The structure of the five molecular species is shown in their non-reduced (left, red) and reduced (right, green) form.
Mal, Suc and Lac are metabolically important sugars that are shorter than most complex carbohydrate structures (glycans) known to carry out wide-ranging biological functions typically ranging from 3-15 monosaccharide residues. Since many mono- and disaccharides have identical mass and elemental composition and only differ in their stereochemistry, mass spectrometry (MS) alone fails to accurately profile these molecules; chromatographic separation of these species ahead of the mass analysis is increasingly preferred due to the recognized analytical performance of modern LC-MS/MS instruments. However, the chromatographic separation of Hex and Hex2 isomers on conventional (and MS compatible) liquid chromatography (LC) phases and solvent systems has, to date, proven challenging due to the only subtle, structural differences between the many mono- and disaccharide species that exist in nature. Therefore, the field urgently requires new separation and detection methods for the rapid, robust, and accurate profiling of saccharides.
Porous graphitic carbon (PGC) is an important stationary phase in the separation sciences that retains non-polar analytes similarly to alkyl-bonded, reversed-phase chromatographic materials.5 PGC has also been demonstrated to display a unique retention and selectivity characteristic of polar and charged compounds, such as complex carbohydrates. For this reason, PGC has been extensively used to separate glycan isomers in the emerging field of glycomics.6 While the market-leading PGC LC column has proven useful for the separation of most N- and O-glycans found in eukaryotes, we have experienced that this PGC column fails to consistently retain mono-, di- and trisaccharides, which are therefore overlooked with our glycomics profiling.
The aim of this work was to assess the abilities of the Supel™ Carbon LC column to retain and separate a variety of mono- and disaccharides in their reduced and non-reduced forms.
Experimental
Fru, Mal, Lac and Suc standards as well as sodium borohydride were purchased from Sigma-Aldrich® (Castle Hill, NSW, Australia). In total, 2 mg of each monosaccharide and disaccharide species were separately dissolved in 1 mL Milli-Q® water from which 10 µL aliquots were made. Each aliquot was then dried in a SpeedVac and dissolved in 50 µL Milli-Q® water and PGC-LC-MS analyses were performed separately for each molecular species.
Reductive beta-elimination was used to reduce the compounds using an established protocol.7 In short, reductive beta-elimination was performed by adding 10 µL 1 M sodium borohydride solution (freshly made in 50 mM potassium hydroxide) to each of the aliquots, which were then incubated at 50 °C overnight. After quenching the reaction with 2 µL glacial acetic acid, the reduced samples were desalted on custom-made ion exchange SPE columns. The desalted samples were dried in the SpeedVac™, dissolved in 150 µL methanol and dried again to remove any remaining borate from the reduction procedure. Finally, the reduced saccharides were dissolved in 50 µL Milli-Q® water for PGC LC-MS analysis using a high resolution 6538 Q-ToF mass spectrometer (Agilent) connected to an Agilent 1260 HPLC system (see Table 1 for details). The non-reduced saccharides were analysed directly using LC-MS/MS without desalting.
The retention behavior of all compounds was investigated separately on a Supel™ Carbon LC column. The reduced and non-reduced Hex and Hex2 compounds were monitored by performing extracted ion chromatograms (EICs) at m/z 179.15, m/z 181.15, m/z 341.096 and m/z 343.11 corresponding to the molecular mass of the non-reduced and reduced Hex and Hex2 isomers, respectively.
Results & Discussion
The results related to the retention behavior of Glc, Fru, Lac, Mal and Suc on the Supel™ Carbon LC column are shown in Figure 2-17 and Table 2. The individual results are separately discussed below. In short, the generated data show that non-reduced and reduced mono- and disaccharides are all reproducibly retained on the PGC column as demonstrated by three chromatographic runs of all samples.
Reproducible results were obtained of non-reduced Fru (m/z 179.16) as demonstrated by stable retention times (6.85-6.86 min) of triplicate injections of this compound, Figure 2A. Non-reduced Fru was observed as both a monomer (m/z 179.16) and dimer (m/z 359.1) in the mass spectrometer. The Fru dimer is likely a gas-phase product (likely not present in the liquid phase) formed because of the high concentration of the analyte, Figure 2B.
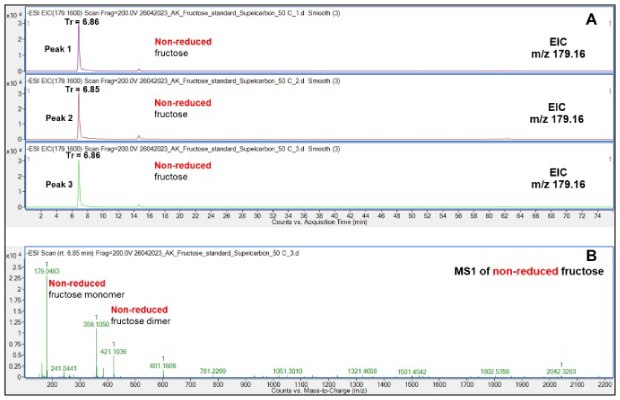
Figure 2.Retention behavior of non-reduced Fru on the Supel™ Carbon LC column as visualized by EICs of monomeric Fru (A) and a representative mass spectrum (B) showing the presence of both a Fru monomer and dimer, the latter a gas-phase product. Numbered peaks (peaks 1-3) are summarized in Table 2.
Reduced Fru eluted later (7.3-7.52 min) than the non-reduced species (6.85 min), Figure 3-4. Despite identical injection amount of the analytes, the peak shapes of the reduced Fru appeared markedly less sharp compared to its non-reduced counterpart perhaps due to saturation of the detector of the reduced Fru which may ionize better than non-reduced Fru.
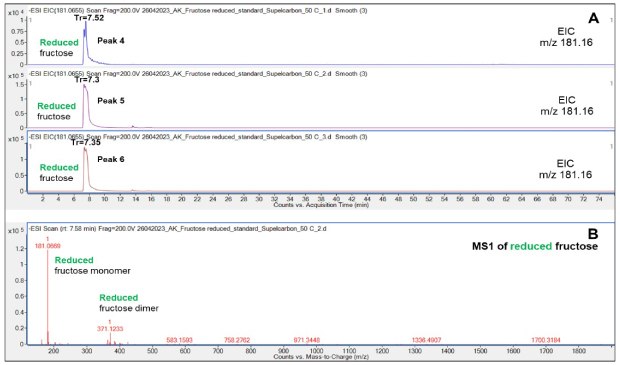
Figure 3.Retention behavior of reduced Fru on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) showing the reduced Fru monomer and dimer. See also Table 2 for overview of all structures and LC retention times of all peaks (peaks 4-6).
The retention time difference between reduced and non-reduced Fru suggests that the formation of an -OH group on the second carbon atom of the linear molecule during reduction (see Figure 1) may enable stronger interactions of reduced Fru with the PGC column resulting in longer retention times. However, the presence of the -OH group might decrease the gas-phase dimer formation demonstrated by the significantly less intense dimer peak for reduced Fru compared to that obtained for non-reduced Fru dimer.
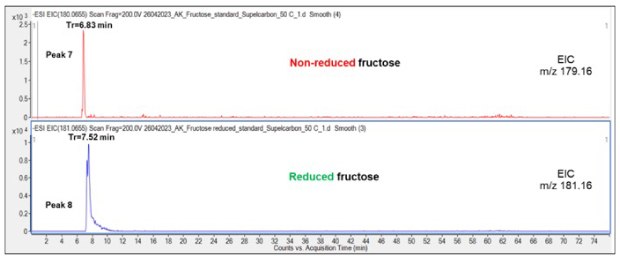
Figure 4.Comparison of the retention pattern of non-reduced and reduced Fru on the Supel™ Carbon LC column. See also Table 2 for overview of all structures and LC retention times of the peaks (peaks 7-8).
Next, comparative analysis of the retention behavior of the Hex monosaccharide isomers was performed to shed light on the role of differently located double bonded oxygen in the two species.
The retention behavior of non-reduced Glc and Fru was firstly compared, Figure 5. Non-reduced Glc (8.32 min) eluted significantly later than non-reduced Fru (6.85 min), a possible result of the presence of a six-membered ring in non-reduced Glc and a five-membered ring in non-reduced Fru and consequently, the location of two hydroxymethyl groups outside of the planar structure of the ketohexose.
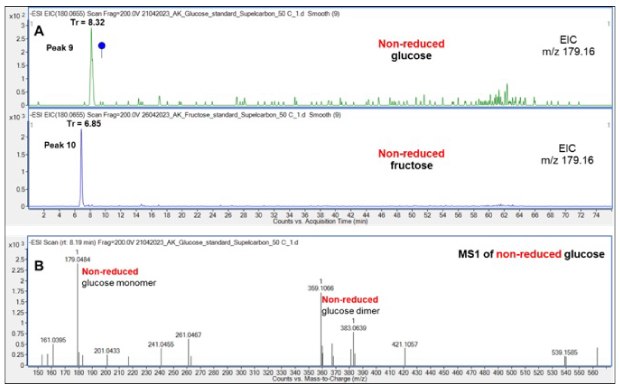
Figure 5.Comparison of the retention of non-reduced Glc and Fru on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) showing the presence of non-reduced Glc monomer (m/z 179.1) and dimer (m/z 359.1). See also Table 2 for overview of all structures and LC retention times of the peaks (peaks 9-10). Blue circle: Glc. Cartoon is depicted according to the latest Symbol Nomenclature for Glycans.8
In contrast, reduced Glc (7.76 min) and Fru (7.52 min) displayed only slightly different elution on the PGC column, Figure 6. This result indicates that the spatial location of the hydroxyl group on the second carbon atom in the elongated form of reduced Mal has minimal effect on the interaction of these two Hex isomers with the PGC column. Analysis of a mixture of reduced Fru and Glc is required to evaluate if these two species can be separated on the PGC column.
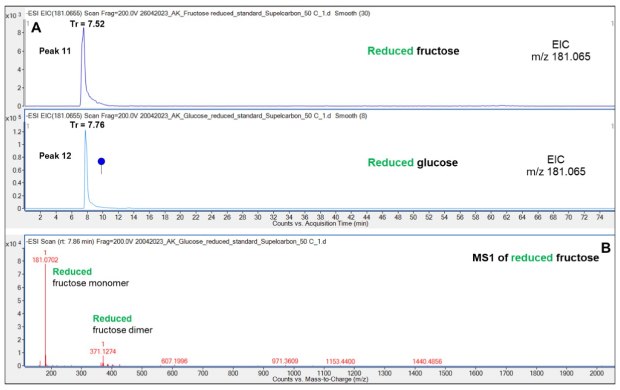
Figure 6.Comparison of the retention pattern of reduced Glc and Fru on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) showing the reduced Fru monomer and dimer. See also Table 2 for overview of all structures and LC retention times of the peaks (peaks 11-12). Blue circle: Glc. Cartoon is depicted according to the latest Symbol Nomenclature for Glycans.8
Chromatographic analysis of the non-reduced and reduced Mal disaccharide was also carried out. Almost identical retention times were repeatedly obtained demonstrating a high reproducibility of the chromatographic runs, Figure 7 and Figure 9, respectively. Both Mal monomers (m/z 341.097) and dimers (m/z 683.20) and even trimers (m/z 1025.31) were observed, but their identical retention times on the extracted ion chromatogram (EIC) support that the oligomers are formed in the gas phase as opposed to in the solution phase, Figure 8.
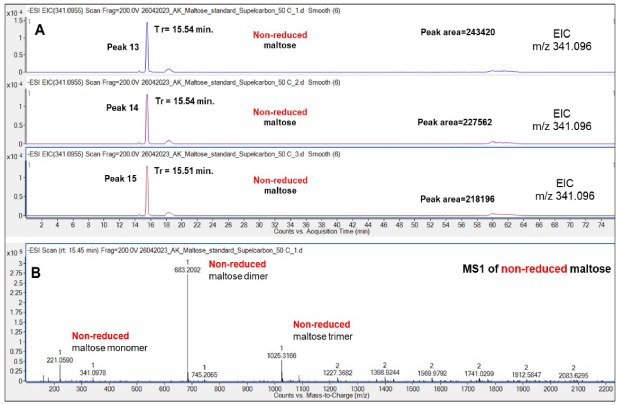
Figure 7.Retention behavior of non-reduced Mal on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) of non-reduced Mal monomer (m/z 341.1), dimer (m/z 683.2) and trimer (m/z 1025.3). Numbered peaks (peaks 13-15) are summarized in Table 2.
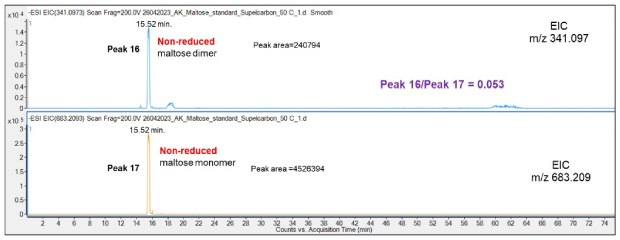
Figure 8.Retention behavior of non-reduced Mal dimer and monomer on the Supel™ Carbon LC column. Numbered peaks (peaks 16 - 17) are summarized in Table 2.
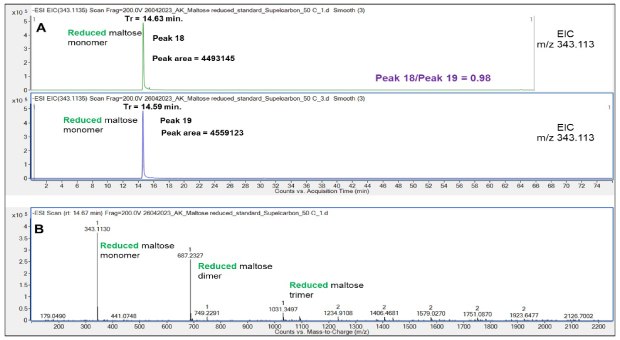
Figure 9.Retention behavior of reduced Mal on the Supel™ Carbon LC column (A) and a representative mass spectrum of reduced Mal (B). Numbered peaks (peaks 18-19) are summarized in Table 2.
Chromatographic runs displayed a shorter retention time of reduced Mal (14.63 min) compared to non-reduced Mal (15.52 min, Figure 10), which can be attributed to the different structure of the truncated and linear form of the non-reduced and reduced disaccharide and their different interaction with the PGC LC column.
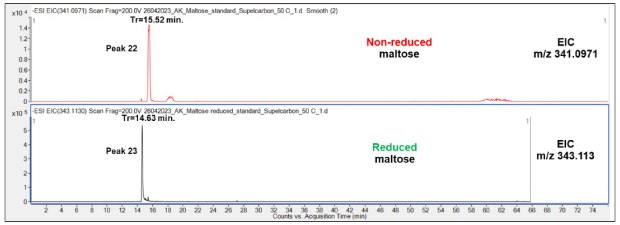
Figure 10.Comparison of retention behavior of non-reduced (top chromatogram) and reduced (bottom) Mal on the Supel™ Carbon LC column. Numbered peaks (peaks 22-23) are summarized in Table 2.
Next, non-reduced Lac was analyzed. While this compound also demonstrated good and reproducible retention, relatively broad peaks were observed at 15.53 min as a likely result of detector saturation either due to column overloading or better ionization properties (relative to the other saccharides) of this compound, Figure 11.
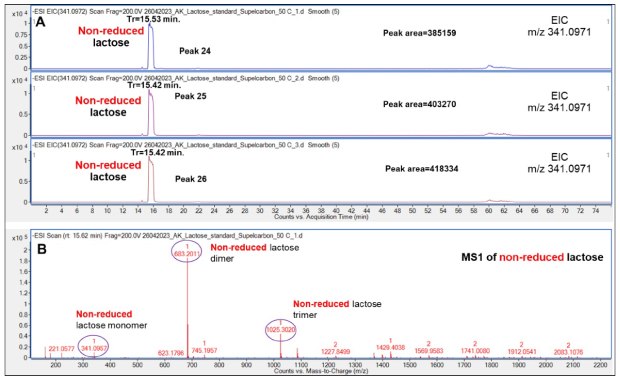
Figure 11.Retention behavior of non-reduced Lac on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) showing a non-reduced Lac monomer, dimer and trimer. Numbered peaks (peaks 24-26) are summarized in Table 2.
Near-identical retention times were obtained from triplicate experiments with reduced Lac (14.4 min) demonstrating the reproducibility of the analysis and the efficacy of the Supel™ Carbon LC column, Figure 12.
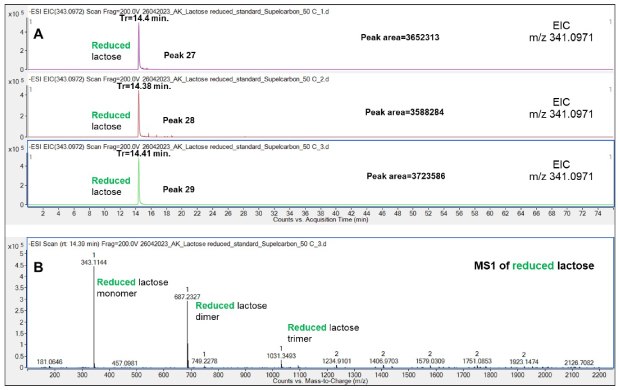
Figure 12.Retention behavior of reduced Lac on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) demonstrating that reduced Lac monomer, dimer and trimer form in the gas phase. Numbered peaks (peaks 27-29) are summarized in Table 2.
Similarly, to the observations made with the Mal experiments, the retention time of reduced Lac (14.4 min) was found to be shorter than the non-reduced counterpart (15.54 min) indicating a stronger interaction of the non-reduced compound with the PGC material, Figure 13. The peaks for reduced Lac were markedly sharper than for non-reduced Mal providing an opportunity for better separation of the reduced compound.
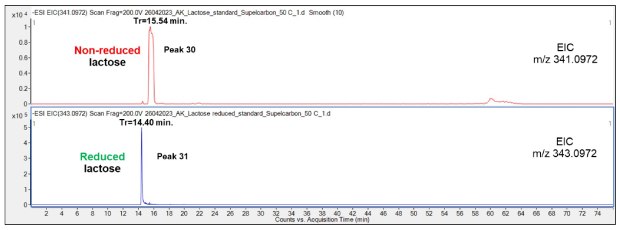
Figure 13.Comparison of the retention behavior of non-reduced (top chromatogram) and reduced (bottom) Lac on the Supel™ Carbon LC column. Numbered peaks (peaks 30-31) are summarized in Table 2.
Triplicate chromatographic runs were also performed of non-reduced Suc, which demonstrated a highly reproducible retention (15.33-15.36 min) on the Supel™ Carbon LC column, Figure 14.
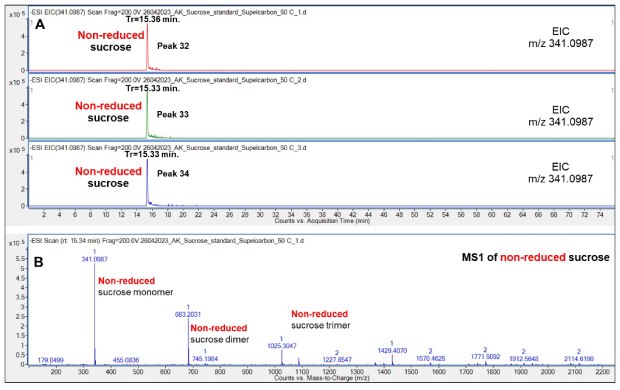
Figure 14.Retention behavior of non-reduced Suc on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) demonstrating the presence of a non-reduced Suc monomer, dimer and trimer in the gas-phase. Numbered peaks (peaks 32-34) are summarized in Table 2.
As expected, the sodium borohydride treated “reduced” Suc (15.32 min) demonstrated near-identical retention as the non-reduced Suc (15.33 min) since Suc does not possess a reducible chemical group, Figure 15.
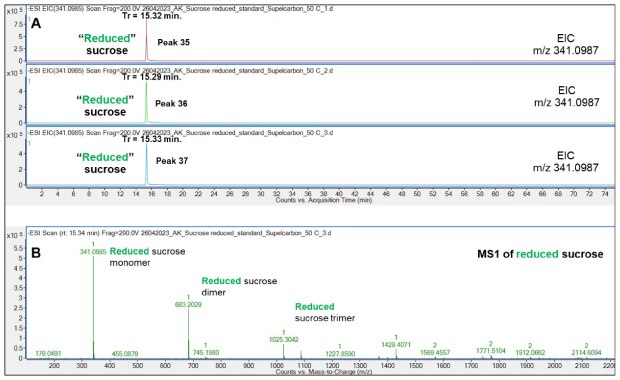
Figure 15.Retention behavior of sodium borohydride treated “reduced” Suc on the Supel™ Carbon LC column (A) and a representative mass spectrum (B) demonstrating the presence of “reduced” Suc monomers, dimers and trimers in the gas phase. Numbered peaks (peaks 35-37) are summarized in Table 2.
Finally, comparative analysis of the retention behavior of the Hex2 disaccharide isomers were performed.
The comparison of the non-reduced Mal, Lac and Suc revealed that the three disaccharides eluted with similar retention time, Figure 16. However, the analysis of a mixture of the three analytes are required to test if the non-reduced Suc may be separated from the slightly later eluting non-reduced Lac and Mal species.
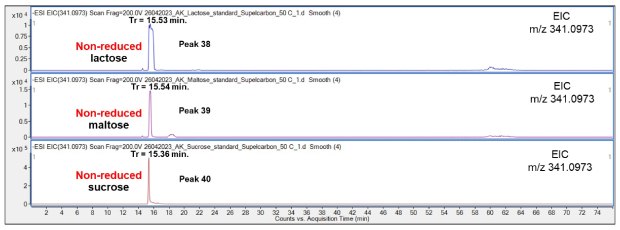
Figure 16.Comparison of retention behavior of non-reduced Lac (top chromatogram), Mal (middle) and Suc (bottom) on the Supel™ Carbon LC column. Numbered peaks (peaks 38-40) are summarized in Table 2.
In contrast, the comparison of the three reduced disaccharide species demonstrated a potential for their separation, Figure 17. The prolonged retention time for Suc relative to reduced Lac and Mal is likely because Suc does not contain chemical groups that are sensitive to sodium borohydride.
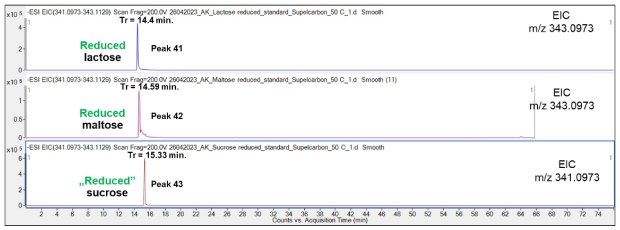
Figure 17.Comparison of the retention behavior of reduced Lac, Mal and sodium borohydride treated “reduced” Suc on the Supel™ Carbon LC column. Numbered peaks (peaks 41-43) are summarized in Table 2.
The peaks and corresponding structures observed throughout the conducted experiments underpinning this report have been summarized in Table 2.
Conclusion
We have here investigated the retention behaviour of non-reduced and reduced forms of two Hex monosaccharide isomers (Fru, Glc) and three Hex2 disaccharide (Mal, Lac and Suc) isomers on a Supel™ Carbon LC column. We have demonstrated that the Supel™ Carbon LC column effectively retains both the non-reduced and reduced forms of all mono-and disaccharides in a reproducible manner and generally with good peak characteristics (peak symmetry, narrow peak width).
Reduced Fru exhibited increased retention compared to non-reduced Fru while the opposite was observed for Glc, i.e. reduced Glc eluted earlier than non-reduced Glc. Excitingly, non-reduced Glc and Fru were well separated by approximately 1.5 min demonstrating the powerful separation potential of the PGC column. In contrast, reduced Fru and Glc exhibited similar retention behaviour suggesting that their subtle structural differences have little impact on the PGC retention mechanism in their reduced form.
Reduced Mal and Lac eluted nearly one minute earlier than their corresponding, non-reduced disaccharides demonstrating that these species can be effectively separated using the Supel™ Carbon LC column. In contrast, only relatively poor separation was achieved for both the reduced and the non-reduced forms of Mal and Lac under the tested conditions. The analysis of their mixtures is required to confirm this preliminary conclusion. Follow-up experiments utilising different solvent and gradient conditions are under way in attempts to improve further the separation of Fru and Glc as well as Mal, Lac and Suc as the separation of such mono- and disaccharide isomers would be of high interest to advance the field of glycomics and analytical carbohydrate chemistry.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?