Macmillan Imidazolidinone Organocatalysts
Metal-free Asymmetric Catalysis
Developed by Professor David MacMillan at Caltech, imidazolidinone-based OrganoCatalysts are designed to serve as general catalysts for a variety of asymmetric transformations. The first highly enantioselective organocatalytic Diels-Alder reaction using (5S)-2,2,3-trimethyl-5-phenylmethyl-4-imidazolidinone monohydrochloride was reported by MacMillan in his pioneering work in 2000 (Scheme 1).1 The activated iminium ion, formed through condensation of the imidazolidinone and an α,β-unsaturated aldehyde, underwent reaction with various dienes to yield [4+2]-cycloadducts in excellent yields and enantioselectivities.
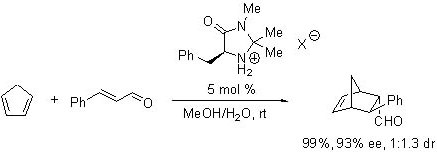
Scheme 1.
Other organocatalytic transformations such as 1,3-dipolar cycloadditions,2 Friedel-Crafts alkylations,3 α-chlorinations,4 α-fluorinations,5 and intramolecular Michael reactions6 were reported using MacMillan’s Imidazolidinone OrganoCatalysts, all proceeding with high levels of enantioselectivity (Scheme 2).
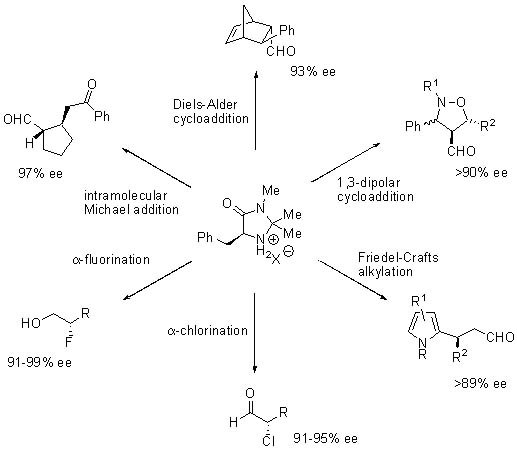
Scheme 2.
MacMillan found an optimized structure in (2S,5S)-(−)-2-tert-butyl-3-methyl-5-benzyl-4-imidazolidinone for the Friedel-Crafts alkylation of indoles (Scheme 3). 6,7
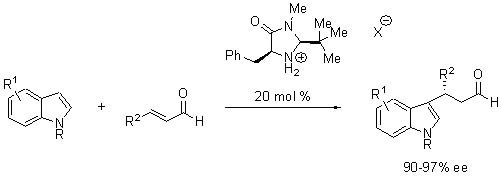
Scheme 3.
The synthetic utility of this concept was later demonstrated in the total synthesis of (–)-flustramine B, a biologically active pyrroloindoline-containing alkaloid (Scheme 4).
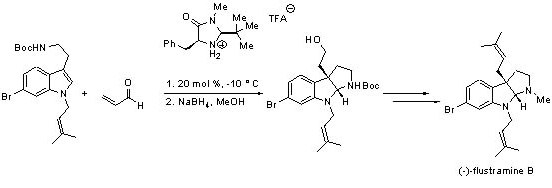
Scheme 4.
References
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?