Evaluation of Different Enzymes on Hydrolysis Efficiencies of Glucuronide Drug Metabolites in Urine
Jim Blasberg, Harkewal Singh, Kevin Ray
Sigma-Aldrich, St. Louis, MO
Introduction
β-glucuronidase (GUS) enzymes are utilized to hydrolyze glucuronide (gluc) drug metabolites to the parent drug, facilitating analysis by LC-MS/MS. Here we evaluate the hydrolysis efficiency of β-glucuronidase derived from Patella vulgata (limpet), Helix pomatia (snail), Red abalone, and E. coli on drugs of abuse and pain management in a synthetic urine matrix. Hydrolysis efficiencies vary from product to product and several parameters need to be investigated in determining which enzyme to use.
Background
Dimer of β-GUS (inset: expanded view of active site)
Common Currency for Glucuronidase Activity
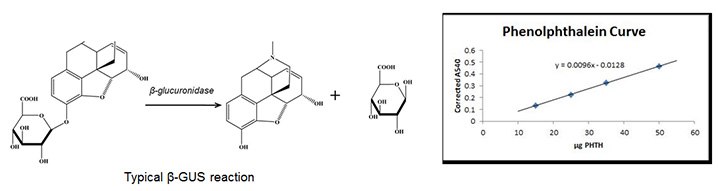
One Fishman1 unit will liberate 1 μg phenolphthalein from phenolphthalein glucuronide per hour at 37οC All hydrolysis experiments were normalized for enzyme activity, i.e. equivalent Fishman units
Methods
- Target glucuronides were spiked into synthetic urine.
- Heavy parent (non-glucuronide) drug was spiked as an internal standard.
- Added enzyme at an appropriate pH, incubated at 60 °C.
- Each time-point pulled sample and precipitate protein with TCA.
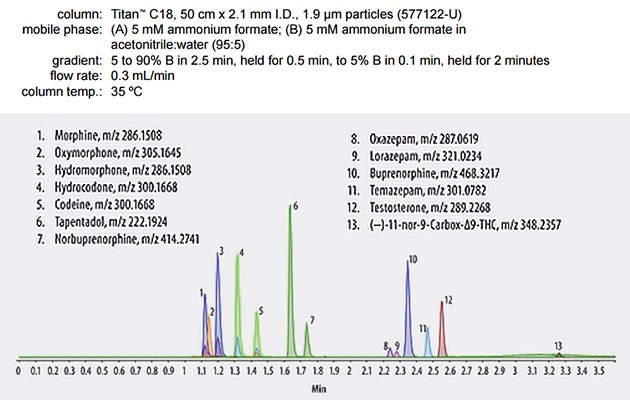
- Analyzed by LC-MS/MS (typical conditions below).
Results
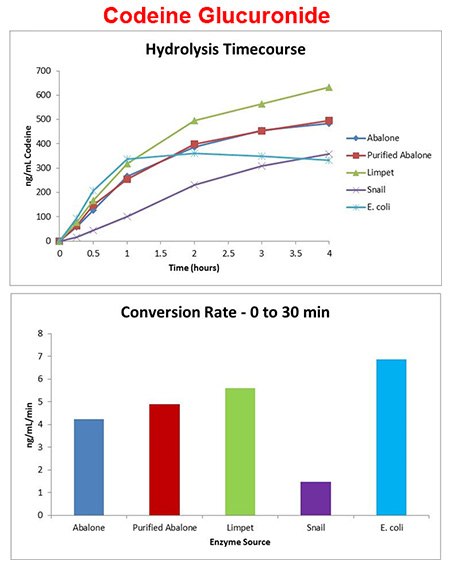
Codeine-gluc enzyme source
- Enzyme added at 10 units/μL of urine and incubated at 60 °C.
- Codeine glucuronide conversion was enzyme source dependent at 10 U/μL, 60 °C.
- E. coli enzyme shows high activity early but appears to lose activity after 1 hour at 60 °C.
- Limpet enzyme shows >95% codeine glucuronide conversion at 10 U/μL, 60 °C.
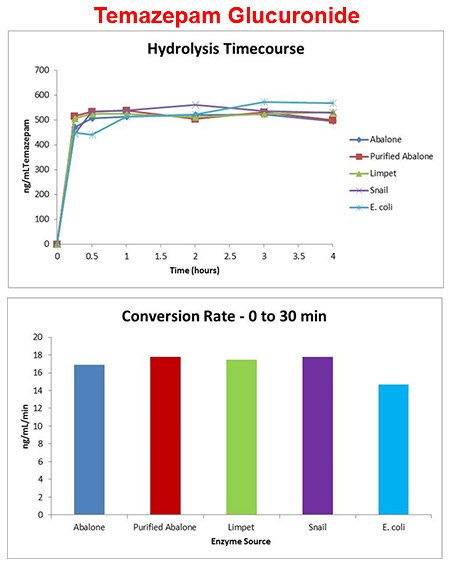
Temazepam-gluc enzyme source
- Enzyme added at 1 unit/μL of urine and incubated at 60 °C.
- Temazepam glucuronide conversion was fast at 1 U/μL, 60 °C, for all enzymes tested.
- Most benzodiazepine glucuronides show full conversion even at low titer and 0.5 hr @ 60C
Purified Abalone GUS
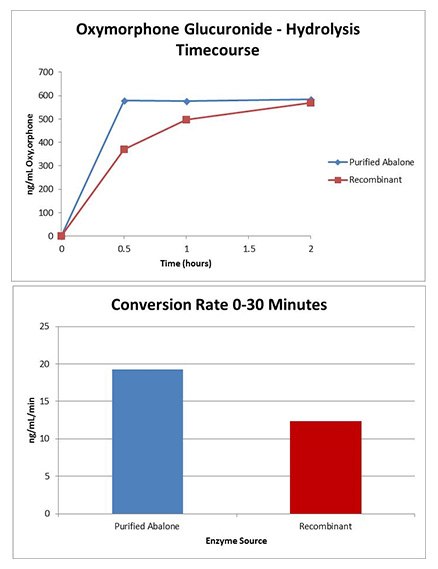
- Oxymorphone glucuronide hydrolysis was equivalent at 10 U/μL, 60 °C, for purified abalone GUS and recombinant GUS.
Recombinant GUS Comparison - 6MAM Conversion
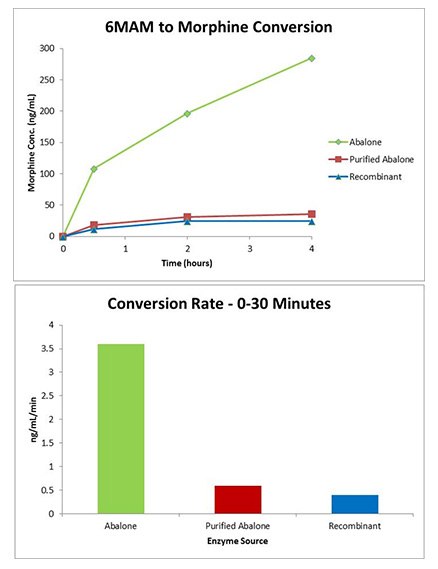
- Traditional abalone exhibited substantial 6-MAM to morphine conversion.
- 6-MAM conversion at 75 U/μL, 60 °C, was equivalent for purified abalone GUS and recombinant GUS.
Effect of Enzyme Titer - Purified Abalone
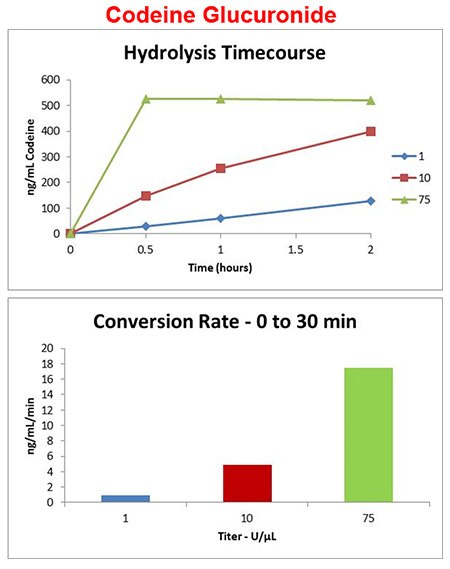
Codeine-gluc enzyme titer
- Purified abalone enzyme added at 1, 10, and 75 units/μL of urine and incubated at 60 °C.
- Enzyme titer greatly affects conversion rate
- Hydrolysis of codeine glucuronide completes in less than an hour at 75 U/μL.
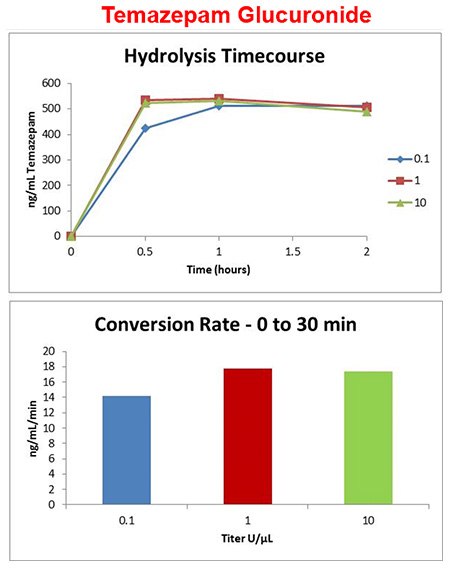
Temazepam-gluc enzyme titer
- Purified abalone enzyme added at 0.1, 1, and 10 units/μL of urine and incubated at 60 °C
- Temazepam glucuronide conversion was less dependent on enzyme titer.
- Hydrolysis of temazepam glucuronide completes in less than an hour at 1 U/μL.
Conclusions
- Effect of Enzyme Source
- Glucuronide conversion efficiency is enzyme-dependent.
- Glucuronide conversion efficiency is also substrate-dependent.
- Effect of Enzyme Titer
- At high titer even difficult to convert substrates, such as codeine, are hydrolyzed rapidly.
- In general benzodiazepines, such as temazepam, hydrolyze rapidly even at low titers.
- Comparison of Purified Abalone and Recombinant GUS
- Purified abalone hydrolyzed oxymorphone glucuronide quickly at 10 U/μL, comparable to recombinant GUS.
- Purified abalone induced minimal 6-monoacetyl morphine (6-MAM) conversion even at high titer.
Materials
Reference
계속 읽으시려면 로그인하거나 계정을 생성하세요.
계정이 없으십니까?